Fibrin patch
A strategy carried out by our group was the use of fibrin glue as a natural patch system to regenerate the damaged myocardium. Fibrin has the ability to serve as biological glue, holding cells together in site as well as stimulating angiogenesis thus, it could be a good vehicle for therapeutic cells implantation.
In a first study, we designed an in vivo experiment in which we used fibrin glue for implantation of human umbilical cord blood mesenchymal stem cells (UCBMSCs) labelled with a non-invasive bioluminescence imaging (BLI) system and fluorescence. BLI allows real-time monitoring of the location, proliferation, and differentiation of luciferase-expressing cells in living tissues. First, we labelled the cells with this system, loaded them onto a three-dimension (3-D) fibrin patch and transplanted to cover injured myocardium in an immunodeficient mouse model of myocardial infarction (MI). As a result, implanted cells showed early proliferation and differentiation within the construct in the post-infarcted animals in the MI-UCBMSC group. The implanted cells also participated in the formation of new, functional microvasculature that connected the fibrin-cell patch to both the subjacent myocardial tissue and the host circulatory system. Importantly, treated animals improved their cardiac function and showed a reduced infarct scar. In summary, a 3-D engineered fibrin patch comprised of UCBMSCs attenuated infarct-derived cardiac dysfunction when transplanted locally over a myocardial wound.
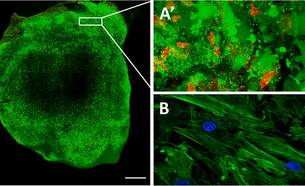
Roura S, Bagó JR, Soler-Botija C, Pujal JM, Gálvez-Montón C, Prat-Vidal C, Llucià-Valldeperas A, Blanco J, Bayes-Genis A. Human umbilical cord blood-derived mesenchymal stem cells promote vascular growth in vivo. PLoS One. 2012;7(11):e49447.
Roura S, Soler-Botija C, Bagó JR, Llucià-Valldeperas A, Férnandez MA, Gálvez-Montón C, Prat-Vidal C, Perea-Gil I, Blanco J, Bayes-Genis A. Postinfarction Functional Recovery Driven by a Three-Dimensional Engineered Fibrin Patch Composed of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Stem Cells Transl Med. 2015 Aug;4(8):956-66.
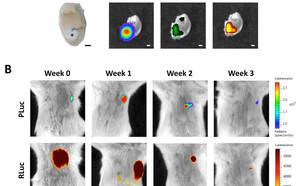
In the same line, we also transplanted human cardiac and subcutaneous adipose tissue-derived progenitor cells (ATDPCs) embedded onto a fibrin patch in the immunodeficient mouse model of MI. In this case, our goal was to monitor in vivo cardiac and endothelial differentiation of cardiac ATDPCs via BLI. Light levels were quantified every week and animals were sacrificed at one month post-implantation. Initial BLI quantification indicated that cardiac differentiation was already induced one week after cell implantation in both cell types, though the levels were remarkably higher in cardiac ATDPCs. Endothelial differentiation was similar in cardiac and subcutaneous ATDPCs, though cardiac cells induced vessel formation more effectively. Accordingly, ATDPC treatment translated into detectable functional and morphological improvements in heart function. In conclusion, ATDPCs differentiate to cardiomyogenic and endothelial lineages when implanted as a fibrin patch in a mouse model of MI, being cardiac ATDPCs more readily to the cardiomyogenic lineage than subcutaneous ATDPCs.
Bagó JR, Soler-Botija C, Casaní L, Aguilar E, Alieva M, Rubio N, Bayes-Genis A, Blanco J. Bioluminescence imaging of cardiomyogenic and vascular differentiation of cardiac and subcutaneous adipose tissue-derived progenitor cells in fibrin patches in a myocardium infarct model. Int J Cardiol. 2013 Nov 15;169(4):288-95.
To further improve the use of cardiac ATDPCs for cardiac regeneration purposes, we developed a device to electromechanically condition these cells in collaboration with Dr. Bragós’s team (Polytechnics University of Catalonia). We hypothesized that in vitro electromechanical stimuli could benefit further cell integration and retention into the heart. Once cells were electromechanically stimulated, they were fluorescence labeled, embedded in the fibrin glue, and implanted over the infarcted area of the mouse heart. Electromechanical conditioning of the cells promoted a cardiogenic-like phenotype in vitro, which drove cardiac function recovery and increased vessel density in the infarct border region when tested in the mouse model. Trained cells within the implanted fibrin patch migrated into the underlying infarcted tissue. To conclude, synchronous electro-mechanical cell conditioning prior to delivery may be a preferred alternative when considering strategies to heart repair after myocardial infarction.
Llucià-Valldeperas A, Soler-Botija C, Gálvez-Montón C, Roura S, Prat-Vidal C, Perea-Gil I, Sanchez B, Bragos R, Vunjak-Novakovic G, Bayes-Genis A. Electromechanical Conditioning of Adult Progenitor Cells Improves Recovery of Cardiac Function After Myocardial Infarction. Stem Cells Transl Med. 2017 Mar;6(3):970-981.
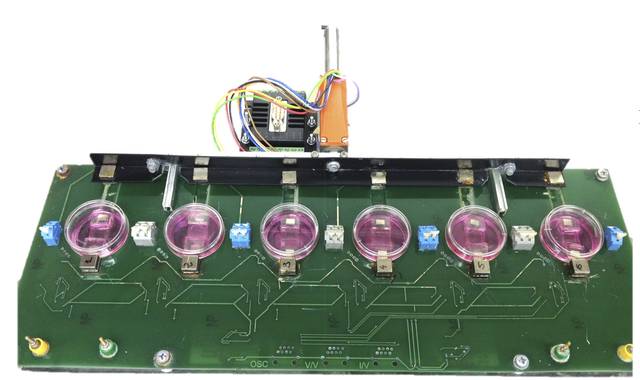
Despite the significance of our results using fibrin patch as a therapeutic cells vehicle and cell differentiation in the host tissue, complete organ regeneration was not well achieved. This suggests the need for more sophisticated therapeutic platforms that limit the spread of the infarcted area and prevent excessive remodelling of the ventricle.